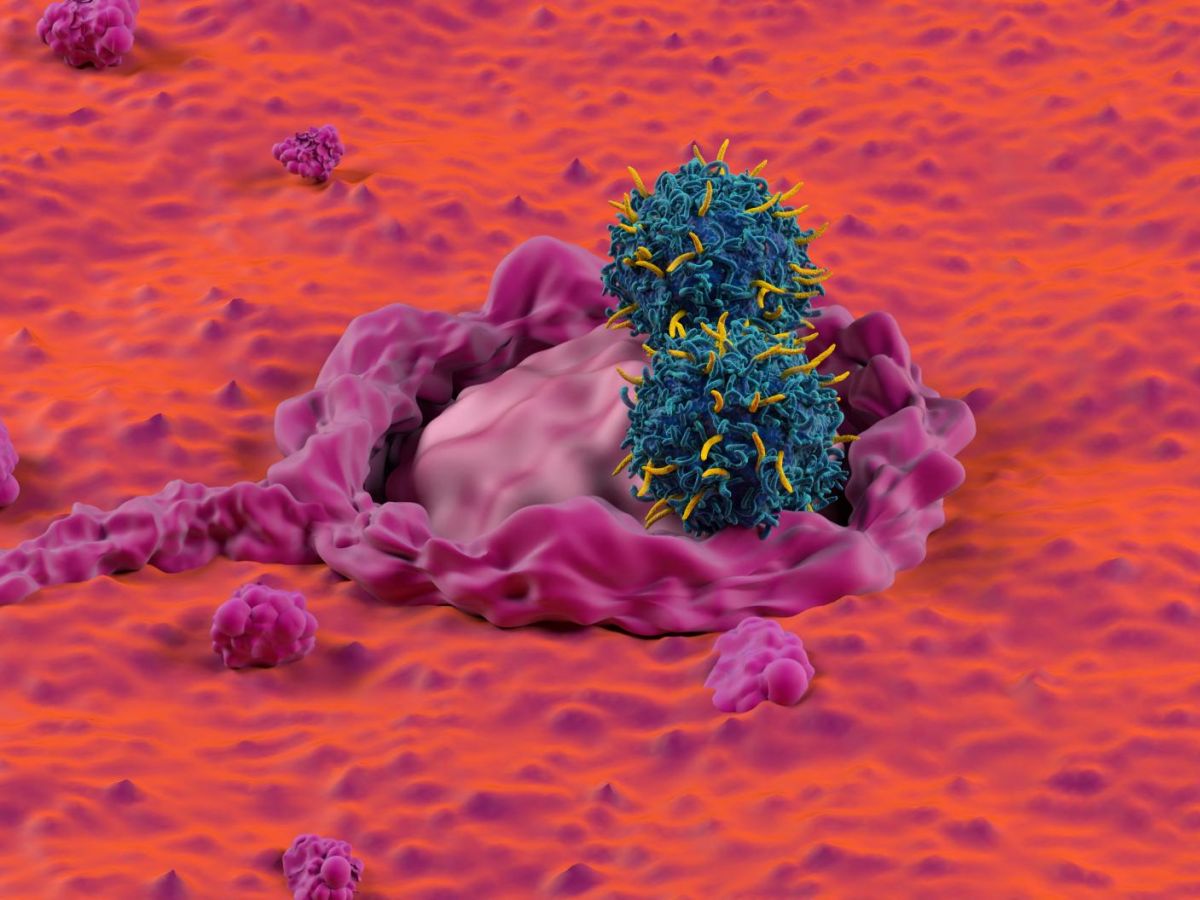
Transforming patients' immune cells to target cancerous tumors is the principle of a cell therapy that has been developing considerably in recent years. These are called CAR-T cells. Their effectiveness had already been proven in previous research on leukemia, notably in 2019.
More recently, it has been demonstrated on cancer cells in the brain. In a new study from Stanford School of Medicine, scientists administered these CAR-T cells to children with deadly brain and spinal cord tumors. We have found a treatment that can shrink tumors and even improve symptoms, " rejoices Michelle Monje, first author of the study. Their results were published in the prestigious journal Nature.
The Mortal Cancers of Infants
Eleven young patients participated in this study. All had brainstem invasive glioma (DIPG) or diffuse midline glioma (DMG), brain and spinal cord cancers that primarily affect children and young adults. These rare and particularly aggressive tumors develop in the brain and spinal cord.
For information, DIPG, which affects approximately 50 children per year in France, is one of the most feared pediatric cancers, with an average survival rate of only 11 months. These cancer cells multiply and invade vital nerve structures. Their diffuse infiltration affects essential functions including motor skills, speech, swallowing, and breathing.
“ These cancers are extremely difficult to treat because of their location and aggressiveness., deplores to Science and Future Michelle Monje, pediatric neuro-oncologist at Stanford (USA). The study of CAR-T cell therapy, which specifically targets these tumors, therefore represents considerable hope. But how do these enhanced cells work?
Read alsoA common virus causes a serious brain cancer, glioblastoma
CAR-T lymphocytes, these immune super-cells
This method relies on immune cells naturally present in our bodies: T lymphocytes. These are key cells of the immune system, capable of recognizing and destroying infected cells. But since cancer cells originate from our own cells, they sometimes slip through their nets.
To address this shortcoming, scientists discovered a revolutionary method in the 1990s: modifying T lymphocytes to specifically target certain cells. These modified lymphocytes are called CAR-T cells. The principle is as follows: researchers collect T lymphocytes from patients and modify them so that they identify a distinctive sign of cancer cells. Once this skill is acquired, the lymphocytes are introduced into the patients' bodies and can attack the cancer cells. Here, the distinctive sign is GD2, a small molecule present on the surface of cancer cells, a prime target for CAR-T cells because it is widely expressed.
Read alsoCAR-T cell treatment that could eradicate blood cancers
Reduce the size of the tumor until it disappears?
Among the participants, nine showed significant benefits. Their neurological functions were improved: DIPG and gliomas infiltrate and integrate at the synapses of neurons,” says the author. The activity of the nervous system is actually the engine of cancer growth." Gliomas therefore cause a dysfunction of neural circuits, but do not generally destroy them.
“ In this trial, we found that when CAR-T cell therapy eliminated cancer cells, neurological functions recovered quickly,” she adds. For four patients, the tumor shrank by more than half, and for one of them, it even disappeared completely from brain scans. Researchers are hopeful that he will be cured.
Although the treatment is promising, it is cumbersome and has significant side effects. Before receiving the CAR-T cells, participants underwent chemotherapy to prevent their immune systems from attacking the modified cells. The researchers first administered the lymphocytes intravenously. However, several side effects then occurred: fever, low blood pressure, and temporary neurological effects due to inflammation within the tumor.
Patients who benefited from the first injection then received two additional injections, directly into the cerebrospinal fluid. This time, the patients experienced fewer side effects. They were able to receive further regular injections.
“ The results obtained in this cohort are very encouraging. However, we still have a long way to go to achieve complete and lasting responses for all patients,” says Michelle Monje. Today, their work aims to understand the disparities in the results of this treatment: why did some patients not benefit from it? The study of biological differences that could explain these disparities in therapeutic response has generated hypotheses that we are currently testing in the laboratory, with the aim of optimizing this therapy in the future," she concludes.