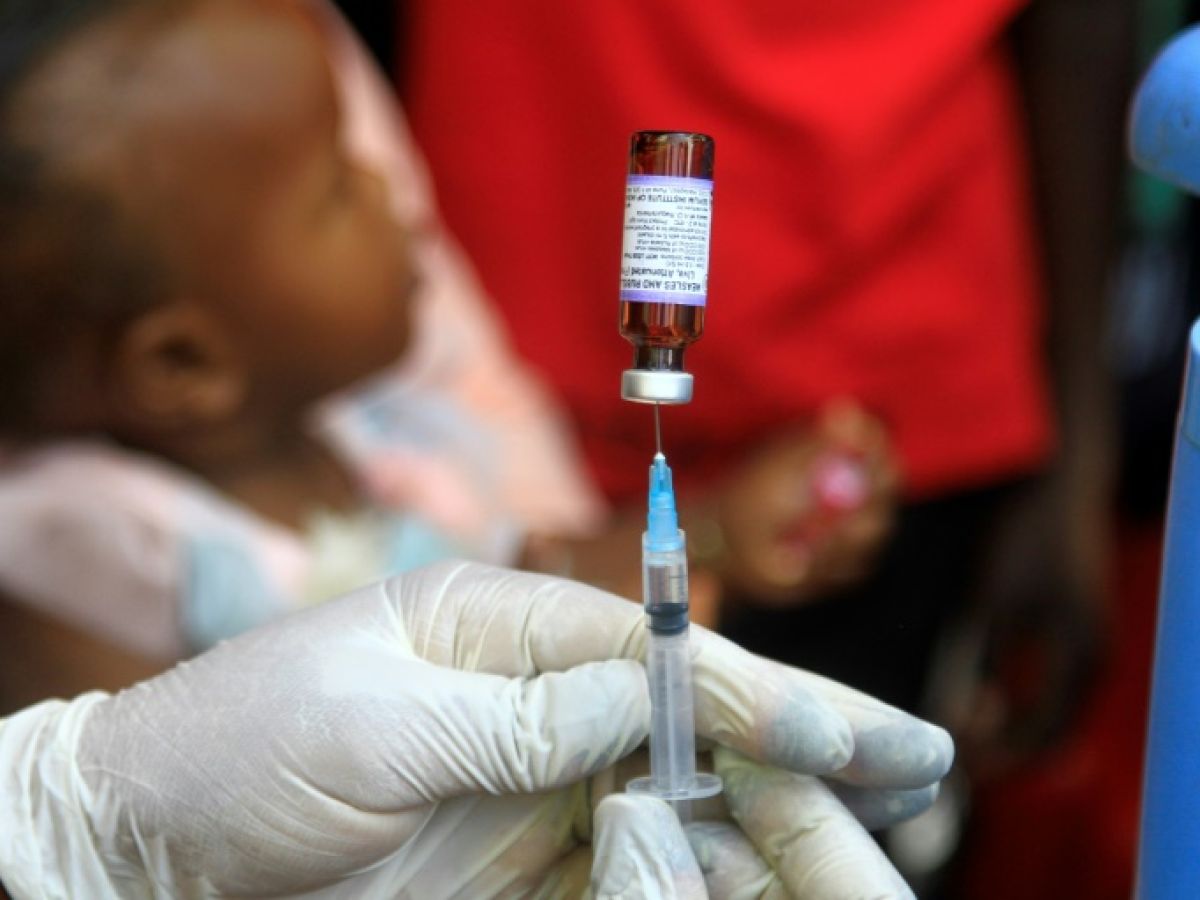
Food giant Danone announced on Friday that it was extending its recall of infant formula to other markets after Singapore, including France, where authorities assured that all batches affected by possible bacterial contamination had been withdrawn from the market.
"In light of new recommendations from a European authority, and as a precautionary measure, we are voluntarily recalling two very limited batches of the following products": Gallia Calisma Relais 1st stage (0-6 months), 830g box – best before date: 13/10/2026 – EAN code: 3041091725943; Blédilait 1st stage (0-6 months), 400g box – best before date: 29/10/2026 – EAN code: 3041091470966," according to a statement sent to AFP.
"In order to comply with the latest recommendations" of certain countries, "Danone will withdraw, in certain targeted markets, a very limited number of specific batches of infant milk," the group said in a statement Friday evening.
A source close to the matter mentioned "the evolution of recommendations from certain authorities, in particular Ireland".
Several batches of infant milk marketed in France and internationally, notably by Nestlé and Lactalis, have recently been recalled due to the potential presence of cereulide, a toxin produced by certain bacteria.
Danone is applying "a maximum precautionary principle, motivated by these new regulatory recommendations," added the source close to the matter.
The group asserts that "routine checks and additional targeted analyses" "confirm" that its products "are safe and fully compliant with all applicable food safety regulations."
On Wednesday, Danone's share price plummeted on the Paris Stock Exchange after the Singapore food agency announced it had blocked several pallets of Dumex 1st stage milk batches.
In France, all batches of infant milk affected by possible bacterial contamination have "been withdrawn" from the market, Health Minister Stéphanie Rist assured on Friday.
Two criminal investigations have been opened in Bordeaux and Angers following the recent deaths of two infants who consumed infant milk recalled by Nestlé due to "possible contamination" by a toxic substance linked to the Bacillus Cereus bacterium (cereulide), without any "causal link" established at this time, according to the authorities.
Speaking to parents who give their babies powdered milk, the minister advised on Friday that they should "check" on the "consumer recall" website to see if their formula had been recalled. "If so, you should put it aside and buy another box of formula," she stated on BFMTV.
– Condolences from Nestlé –
On January 5th, Nestlé initiated a large-scale recall of Guigoz and Nidal brand infant formula due to the potential presence of "cereulide" in these products, which are otherwise subject to rigorous controls. This toxic component, produced under certain conditions by a family of bacteria, Bacillus cereus, can cause severe vomiting within hours of consumption.
Although a causal link with the deaths of the two infants has not been established, the Swiss food giant expressed its "most sincere condolences to the bereaved families" on Friday, saying it remains "at the disposal of the authorities" to cooperate with the investigation.
The manufacturer of the arachidonic acid-rich (ARA) oil that could potentially contain cereulide is the Chinese producer Cabio Biotech, according to sources close to the matter.
This company, founded in 2004 and headquartered in Wuhan (eastern China), is the main Chinese producer of this type of oil and supplies many local and international infant formula producers (Nestlé, Danone, among others).
Following Nestlé, the French company Lactalis also announced on Wednesday the launch of a large-scale recall of infant milk in several countries, including France.
In general, food safety controls are particularly strict for highly sensitive products such as infant milk, according to a report by AFP with the global giant of analytical laboratories, the French company Eurofins.
The search for bacteria of the Bacillus cereus family is systematically offered, but cereulide, a toxin produced by certain strains of Bacillus cereus under certain conditions, is not part of the classic controls.
However, according to Eurofins, in the current context, this test is being requested because all players in the dairy products sector in general, and in infant formula in particular, are concerned about the situation.
bur-pan-ito-ned-ngu/jbo/bfi