This article is taken from the monthly Sciences et Avenir n°941-942, dated July-August 2025.
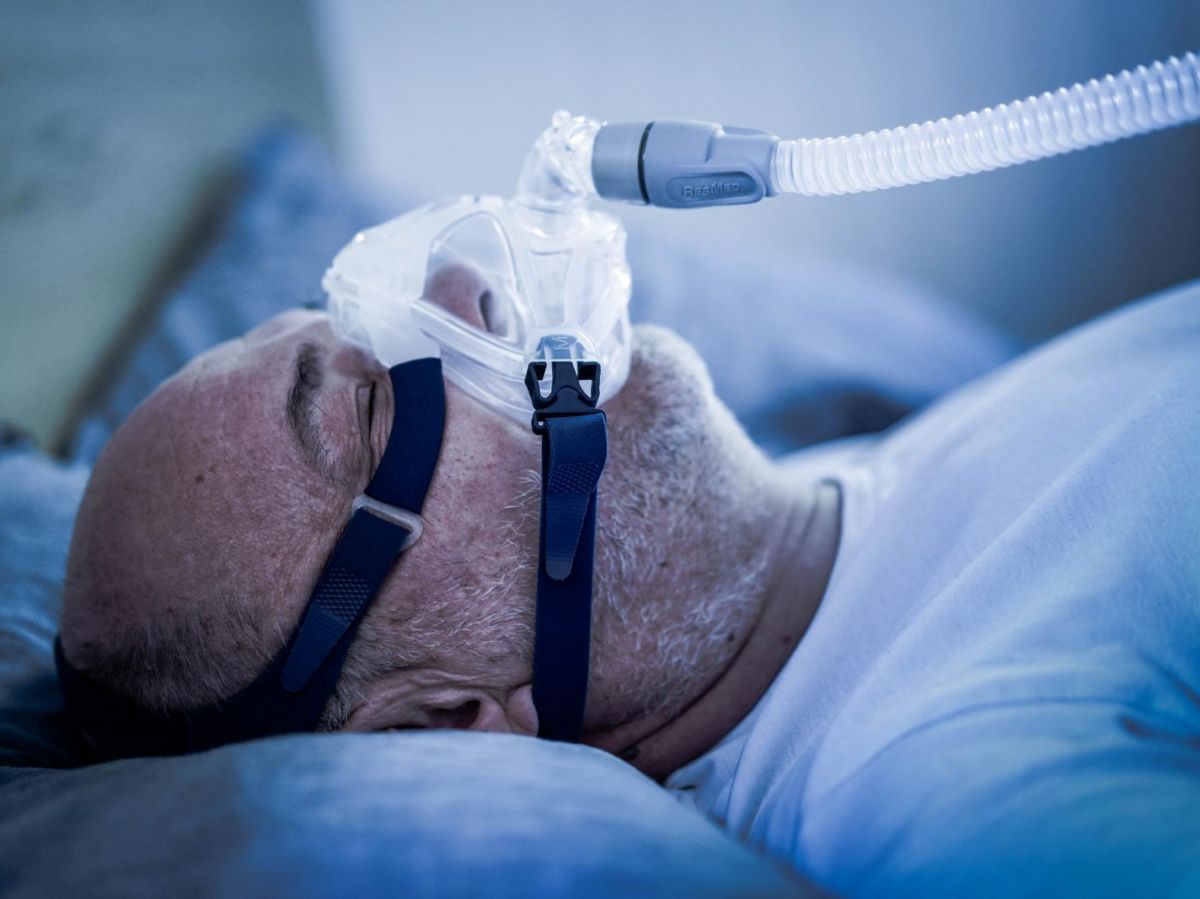
Forced ventilation with a breathing mask worn at night is currently the main treatment for sleep apnea. But a much less restrictive option is emerging with a drug that can reduce or even eliminate these nocturnal breathing interruptions. This was announced by the American startup Apnimed, whose drug solution AD109 is being tested in a clinical trial involving 646 patients. The treatment is based on the combination of two molecules already on the market: one stimulating pharyngeal muscle tone, the other reducing its reduction during sleep.
Disturbed sleep, poor oxygenation and increased strain on the heart
In fact, thesleep apnea Obstructive hypoxia occurs when the relaxation of tissues in the pharynx while lying down blocks the passage of air to the lungs. In addition to loud snoring, it causes disturbed sleep due to micro-awakenings, poor oxygenation of the body and, therefore, greater demands on the heart. In the long term, this hypoxia promotes hypertension, contributes to various cardiovascular pathologies and reduces life expectancy.
Read alsoSleep apnea: a tennis ball can work miracles
A second trial is planned for this summer
According to preliminary results released by the startup, the syndrome was significantly alleviated in 56 % of the cases treated in the clinical trial, and even resolved in 22 % of the patients. Its side effects are dry mouth and insomnia in a quarter of cases. Is this enough to free us from the so-called continuous positive airway pressure (CPAP) mask? "This type of treatment may be of interest to patients who cannot tolerate CPAP, who are very symptomatic, and who do not have severe forms," explains Maxime Patout, an expert pulmonologist at the Pitié-Salpêtrière Hospital in Paris. Because too often, the treatment prescribed to 1.6 million people in France is so poorly tolerated that it is neglected... In any case, a second large-scale trial of AD109 is expected to be completed this summer. And allow Apnimed to file an application for approval with the American drug agency (FDA) by early 2026.