In the Democratic Republic of Congo, the antiviral drug tecovirimat was shown to be safe. However, it did not increase the resolution of clade 1 mpox
August 15, 2024
Press release
Thursday August 15, 2024
An NIH-cosponsored study examined tecovirimat (a microbial syringe) in a country where MPOX is endemic.

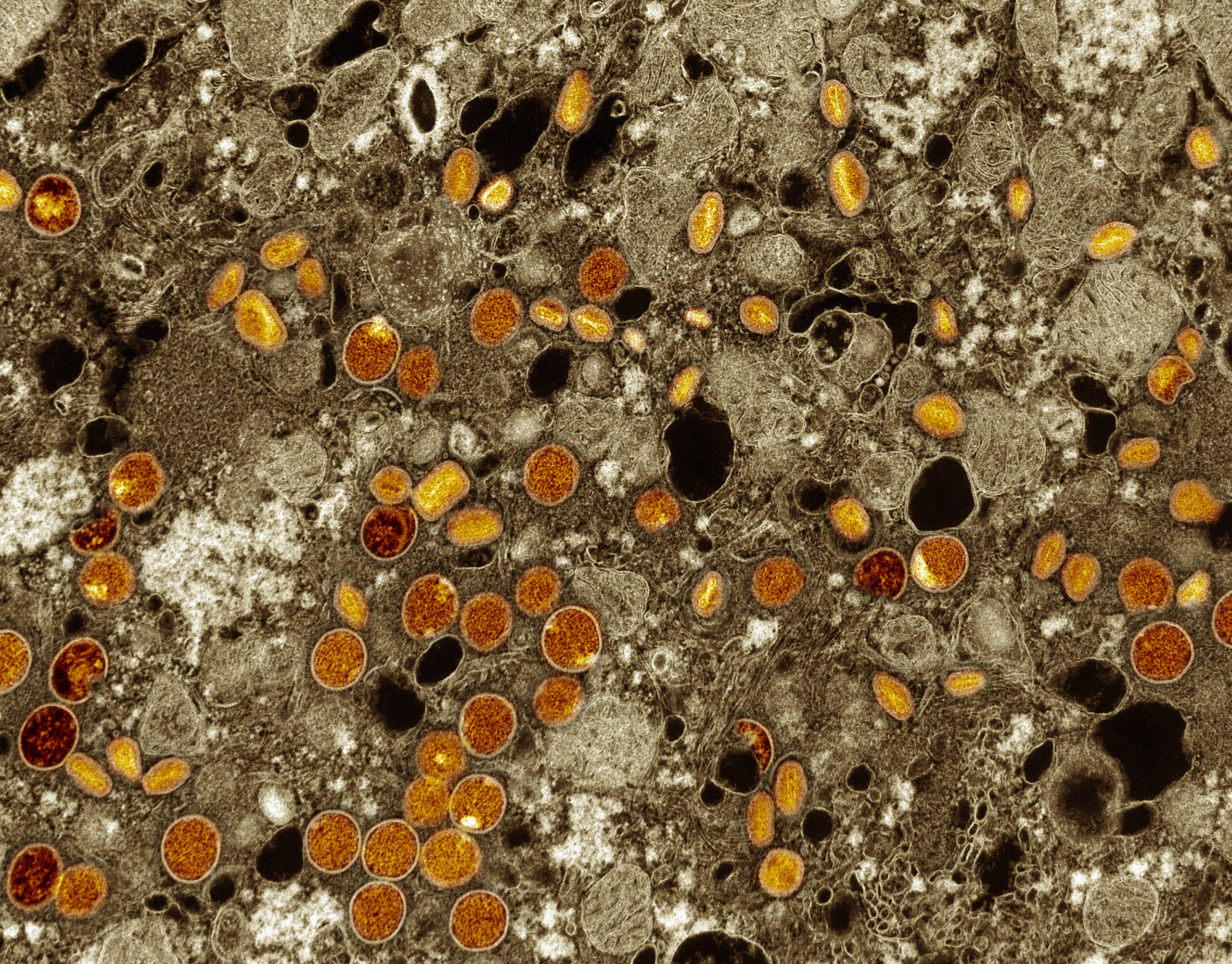
Transmission electron micrograph with colorized MPOX particles (red/yellow), infected VERO cells found (brown). These virus particles are at different stages of maturation, which explains the differences in shape. The NIAID Integrated Research Facility at Fort Detrick, Maryland, captured the virus particles. NIAID
According to an initial analysis of data from a randomized, placebo-controlled trial in the Democratic Republic of Congo, the antiviral drug tecovirimat did not reduce the length of the MPOX lesion in adults and children with clade 1 MPOX. The study found that the overall mortality rate of 1.7% among participants, whether or not they took the drug, was lower than the 3.6% reported in all cases of MPOX. It is clear that people with MPOX benefit from high-quality hospitalization and supportive care. The trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) and co-led through a government partnership with the National Institute of Biomedical Research (INRB) of the DRC. The trial results will be published through scientific channels.
NIAID Director Jeanne Marrazzo, MD, MPH, said, "These results are disappointing but provide important information. They reinforce the need to identify alternative therapeutic candidates for COPD as we continue our research on tecovirimat in other COPD populations." We remain committed to developing safe and effective interventions, including vaccines and treatments, that will help reduce the burden of COPD in Central Africa.
The first case of COPD was identified in West, Central, and East Africa in 1970. There are two types of viruses that cause COPD. Clade I is prevalent in Central Africa and can be severe. Clade II is endemic in West Africa and tends to cause milder symptoms. In 2022, a subtype of clade II caused a worldwide epidemic of COPD. Children, pregnant women, people with weakened immune systems, and those with weakened immune systems are more susceptible to severe COPD, regardless of the virus subtype.
In Central African countries, particularly in the DRC, reports of clade 1 mpox are increasing. The number of cases of clade 1 mpox is increasing in Central African countries, particularly in the DRC. Recent report from the Centers for Disease Control and Prevention The CDC reported that 78 % of suspected MPOX deaths and 67 % of suspected MPOX deaths in the DRC involved children under 15 years of age. TecovirimatTPOXX was developed and initially approved by the Food and Drug Administration for the treatment smallpox The safety and effectiveness of the drug as a treatment for COPD have not yet been established. The drug is available in the United States for the treatment of COPD as part of a separate trial. STOMP, a NIAID-sponsored trial. Through a CDC Expanded Access Investigational New Drugs (EA-IND). Application process. Tecovirimat has been approved in Europe and the United Kingdom to treat smallpox and COPD, as well as other indications.
The next October is 2022. The PALM007 trial was initiated by NIAID in collaboration with INRB To evaluate the safety and efficacy of tecovirimat in the treatment of COPD in adults and children. At two sites in the DRC, 597 patients with laboratory-confirmed cases of COPD were enrolled in the study. Participants were randomly assigned to tecovirimat (or placebo) and admitted for at least 14 days to the hospital, where their safety was closely monitored and lesions resolved. Participants received supportive care, including nutrition, hydration, and treatments for secondary infections.
Tecovirimat did not cause any serious side effects. The overall mortality rate was lower, and lesions healed faster than expected, whether or not participants received Tecovirimat. Study participants will be informed of the preliminary results and will have the opportunity to participate in an extension trial that will provide additional supportive medical care. Further analyses will be conducted to understand the study results. These include determining whether there are differences in clinical outcomes based on the number of days before participants enrolled, the severity of clinical illness, participant characteristics, or genetic variation in the MPOX treated.
This study provided urgently needed evidence on the response to MPOX in Central Africa, said Jean-Jacques Muyembe Tamfum, MD, PhD, Director General of the INRB and Professor of Microbiology at the Faculty of Medicine, University of Kinshasa, Kinshasa, DRC. The results were not what we expected, but they show that the study clinicians provided exceptional support to the participants. This is a testament to their knowledge and skill in managing MPOX-related illness.
“The PALM007 trial demonstrated that robust clinical trials are needed to test the experimental MPOX treatment in an endemic setting,” said Lori Dodd, Ph.D., NIAID PALM project manager for the DRC. “We will continue to evaluate the data from this trial to see if additional studies of Tecovirimat are needed in subgroups of patients.”
Co-principal investigators Muyembe and Tamfum are leading the PALM007 trial. Placide Mbala MD Ph.D. is responsible for operations at PALM Clinical Research Partnership Dr. Véronique Nussenblatt of the INRB is the head of the Department of Epidemiology and Global Health and the Pathogen Genomics Laboratory. Dr. Véronique Nussenblatt of the NIAID Dr. Olivier Tshiani of Leidos Biomedical Research With the help of Congolese staff and the NIH's Frederick National Laboratory for Cancer Research, this trial was conducted in the provinces of Tunda and Kole (Maniema and Sankuru, respectively). The US CDC and the Institute of Tropical Medicine in Antwerp are among the collaborating institutions.The World Health Organization, the Alliance for International Medical Response, and other aid organizations are involved. The U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Embassy in the Democratic Republic of the Congo provided logistical and operational support for the expeditions and travel, as well as regional security. SIGA Technologies, Inc., located in New York City, provided the technology for the study.
In response to the 2018 Ebola outbreak, the Pamoja tulindi Maisha (PALM) Clinical Research Partnership was established. This collaboration continues as a collaborative clinical research program involving NIAID and the DRC Ministry of Health.
The INRB and NIAID would like to thank everyone involved in this study, including the team that conducted it, the Data and Safety Monitoring Board, and, most importantly, the participants and their families. Please visit ClinicalTrials.gov and enter the study number to learn more about PALM007. NCT05559099.
The STOMP study and related research are important to continue, Dr. Marrazzo said, because of the different populations affected by COPD, as well as the clinical diseases that are emerging and their spread.
International STOMP Test The safety and efficacy of tecovirimat are being evaluated against clade 2 MPOX. Please visit ClinicalTrials.gov and enter the study number to learn more about the STOMP trial. NCT05534984. UNITY is an ANRS-sponsored Emerging Infectious Disease study evaluating tecovirimat in Argentina, Brazil, and Switzerland using a study design similar to STOMP. You can find more information about the UNITY trial by searching ClinicalTrials.gov with the study identifier NCT05597735The two studies will work together to continue recruiting participants.
NIAID supports and conducts research at NIH and throughout the United States and around the world to study and develop new methods for diagnosing, preventing, and treating infectious and immune-mediated diseases. On the NIAID website, you can find news releases, fact sheets, and other NIAID materials. NIAID website.
The National Institutes of Health: The NIH is the national medical research agency of the U.S. Department of Health and Human Services. It consists of 27 institutes and centers. The NIH, the national medical research agency, consists of 27 institutes and centers and is part of the U.S. Department of Health and Human Services. Visit the NIH for more information about its programs and services. www.nih.gov.
NIH…Transforming Discovery into Healthcare(r)
###