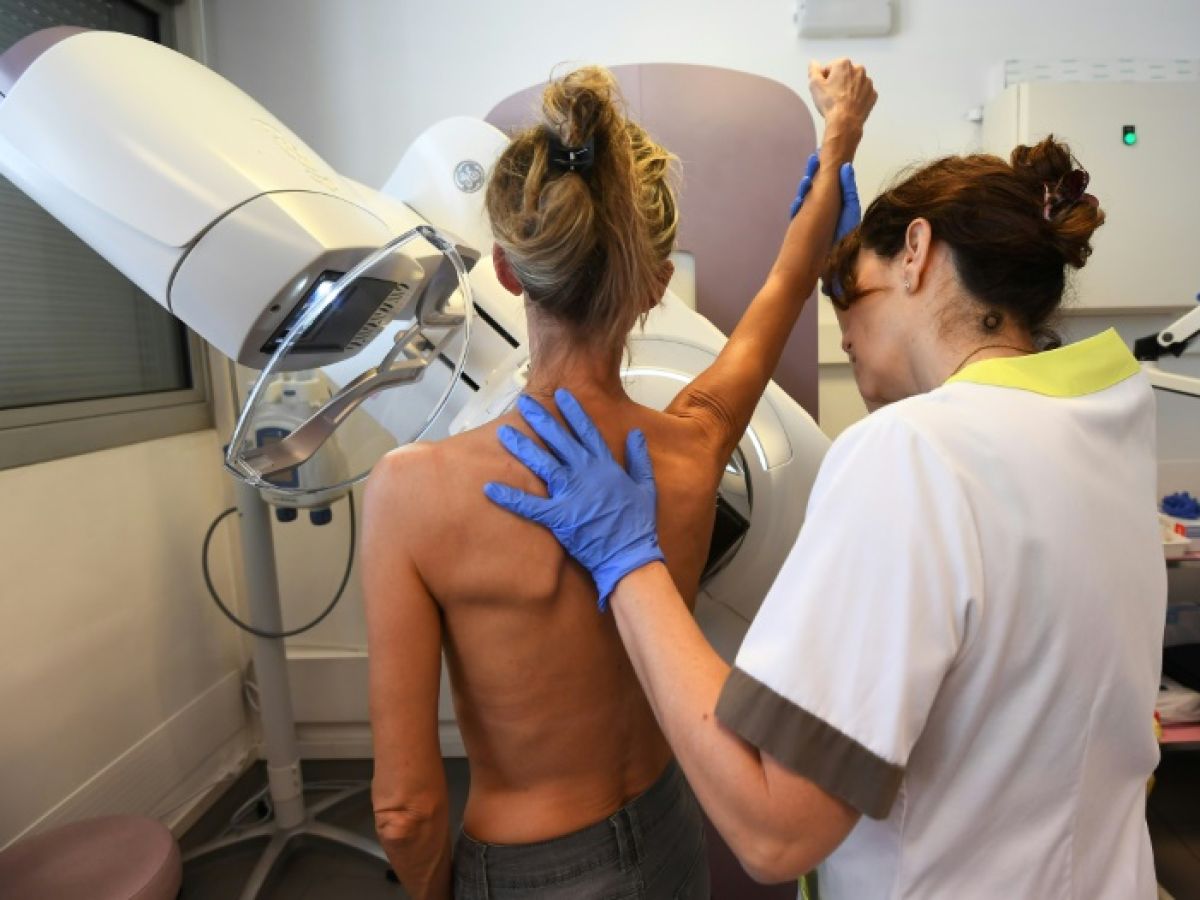
A new treatment halves the risk of progression, or even death, from a form of breast cancer that has not seen significant therapeutic progress for more than a decade, according to a study published Monday.
Scientists presented the results of their study at the annual meeting of the American Society of Clinical Oncology, which are expected to be submitted to regulatory authorities and could soon lead to a new cutting-edge treatment for people with so-called "HER2-positive" metastatic breast cancer, an aggressive form that accounts for 15 to 20% of all breast cancer cases.
These types of cancers are fueled by an overactive HER2 gene, which produces too much of a protein that helps cancer cells grow and spread.
On average, people with this type of cancer, once it has metastasized, have a life expectancy of about five years.
"Seeing such a striking improvement was really impressive for us: we almost doubled the length of time patients could control their cancer," oncologist Sara Tolaney, director of the division of breast oncology at the Dana-Farber Cancer Institute, told AFP.
– “Smart bomb” –
Currently, this type of cancer is treated using a protocol that combines chemotherapy and two antibodies that block the growth signals of the HER2 protein.
The new approach uses another drug (T-DXd), an antibody combined with chemotherapy.

This "smart bomb" strategy allows the drug to directly target cancer cells: "It binds to the cancer cell and allows all of the chemotherapy to be delivered directly to the cancer cells," Tolaney explains.
"Some people call them 'smart bombs' because they deliver the chemo in a targeted way, which is why I think we can significantly increase the effectiveness of the treatment," she continued.
The most common side effects are nausea, diarrhea, and decreased white blood cell count, as well as a less common effect involving lung scarring.
This new treatment is already approved as a second option, used when current treatments no longer work. But in the new trial, it was administered earlier, in combination with another antibody, pertuzumab.
– Duration optimization –
In a large clinical trial led by Sara Tolaney, nearly 400 patients received the new treatment combined with pertuzumab, which is believed to enhance its effects. A similar number of patients received the standard THP treatment.
After two and a half years of follow-up, it appears that the combination of the new treatment (T-DXd) and pertuzumab reduced the risk of disease progression or death in 44% compared to standard treatment.
Better still, 15% of patients in the T-DXd group saw their cancer disappear completely, compared to 8.5% in the standard treatment group.
Because this clinical trial with the new treatment was only temporary, it was possible to subsequently track when half of the patients' cancer recurred or worsened. The average time was 40.7 months with the new treatment, compared to 26.9 months with the standard treatment. This difference could increase further as more data becomes available.
Tolaney emphasizes that the results of her work will be submitted to regulatory authorities around the world, including the U.S. Food and Drug Administration (FDA). Her future research will focus on optimizing treatment duration, particularly for patients in complete remission.