Press release
Monday July 1, 2020
The vaccine candidate could provide broader protection against emerging strains of SARS-CoV-2.

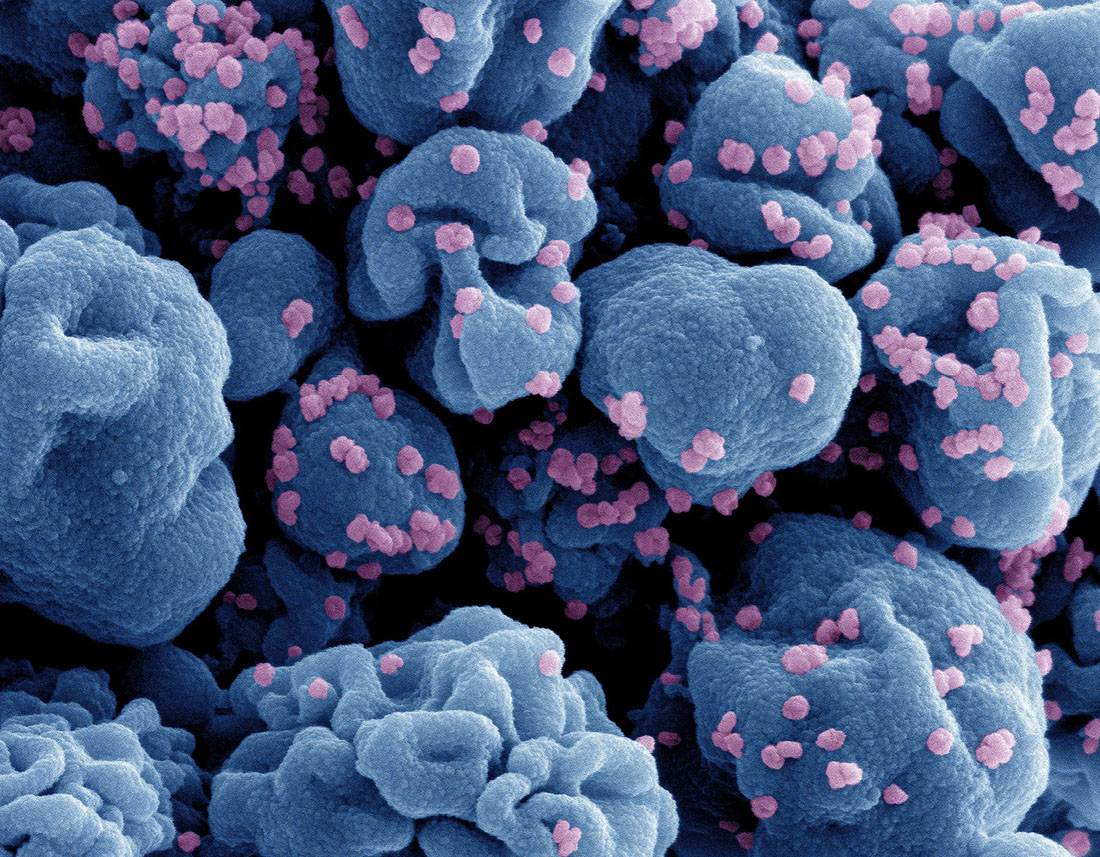
Colorized scanning electron microscope image of an infected cell (in blue), isolated from a sample of SARS-CoV-2 particles. The image was captured by the NIAID Integrated Research Center at Fort Detrick.NIAID
Three sites in the United States are currently recruiting healthy adult volunteers for a Phase 1 study testing the safety and effectiveness of an investigational nasal vaccination that could provide enhanced protection against the COVID-19 virus, which is a variant of SARS-CoV-2. Scientists at the National Institutes of Health's Infectious Diseases Laboratory, National Institute of Allergy and Infectious Diseases, NIAID (NIH), designed and evaluated the vaccine in preclinical trials.
The rapid development of safe and effective vaccines against COVID-19 has been a scientific triumph, and the use of these vaccines has significantly reduced the impact of this pandemic,” said NIAID Director Jeanne M. Marrazzo MDMPH. While first-generation COVID-19 vaccinations are still effective in preventing severe illness, hospitalizations and death, they are less successful in preventing milder illnesses and infections. There is an urgent need for next-generation nasal COVID-19 vaccines that can reduce SARS-CoV-2 infection and transmission.
This study will enroll 60 adults, ages 18 to 64, who have received three or more doses of an FDA-approved or cleared COVID-19 mRNA vaccine. The three trial sites include Baylor College of Medicine in Houston and Hope Clinic in Houston. Emory University Decatur, Georgia and New York University Long Island The study is led by Hana M. El Sahly MD at the Baylor College of Medicine Vaccine Research Center
Three cohorts will be formed from the study volunteers. Study volunteers will receive the lowest possible dose in the form of a nasal spray. Participants in the second and third cohorts will receive progressively larger doses. Scientists will test the tolerance of the vaccine candidate during seven visits spread over a period of approximately one year. They will also see if the nasal spray and blood serum show an immune reaction.
MPV/S-2P is an investigational vaccine that uses the murine pneumonia virus as a vehicle to deliver the SARS-CoV-2 Spike protein (S-2P), stabilized in a prefusion form. The MPV virus does not infect humans or non-human primates, but it has an affinity for the epithelial cells lining the respiratory tract. This could make the vaccine more effective in reaching areas where coronaviruses naturally infect.
The MPV/S-2P vaccine was well tolerated and safe in preclinical studies in non-human primates. The vaccine induced a robust immune response, which included antibodies against SARS-CoV-2 as well as cellular immunity in nasal and respiratory tissues. Mucosal immune responses are more effective than systemic responses in inhibiting the replication of respiratory viruses in humans and animal models.
The U.S. Department of Health and Human Services conducted the first NIAID clinical trial as part of its HHS department. NextGen Project. The NextGen Project, led by the Biomedical Research and Development Authority, part of the HHS Administration for Strategic Preparedness and Response and NIAID, is a collaborative effort between the federal and private sectors to expand the portfolio of innovative new vaccines and therapies. NIAID will facilitate the clinical development of next-generation COVID-19 vaccines through the NextGen Project.
You can find more information about the trial at clinicaltrials.gov Use the identifier NCT06441968.
NIAID supports and conducts research at NIH and throughout the United States and around the world to better understand the causes and develop new methods for diagnosing, preventing, and treating infectious and immune-mediated diseases. On the NIAID website, you can find press releases, fact sheets, and other NIAID materials. NIAID website.
The National Institutes of Health: NIH is the medical research agency of the United States Department of Health and Human Services. It includes 27 institutes and centers. The NIH, the nation's medical research agency, is a component of the U.S. Department of Health and Human Services. It is responsible for conducting basic, translational and clinical medical research and studying causes, treatments and cures. Visit NIH for more information about its programs and services. www.nih.gov.
NIH…Transforming Discovery into Healthcare(r)
###
