There Parkinson's disease is characterized by a progressive loss of dopaminergic neurons, which produce the neurotransmitter dopamine. One of the most characteristic markers of this disease is the accumulation of protein α-synuclein, which forms fibrils and aggregates known as Lewy bodies. However, this accumulation also leads to the appearance of smaller aggregates, oligomers, which are less well studied, but which could play a fundamental role in triggering the disease. According to a study from Aarhus University (Denmark), published on August 12, 2025 in the journal ACS Nano, These small oligomers could embed themselves in the cell membrane of neurons, creating pores that would damage these cells, thus triggering the neuronal loss that causes the disease.
Oligomers ofα-synuclein are attracted bylipids of the cell membrane
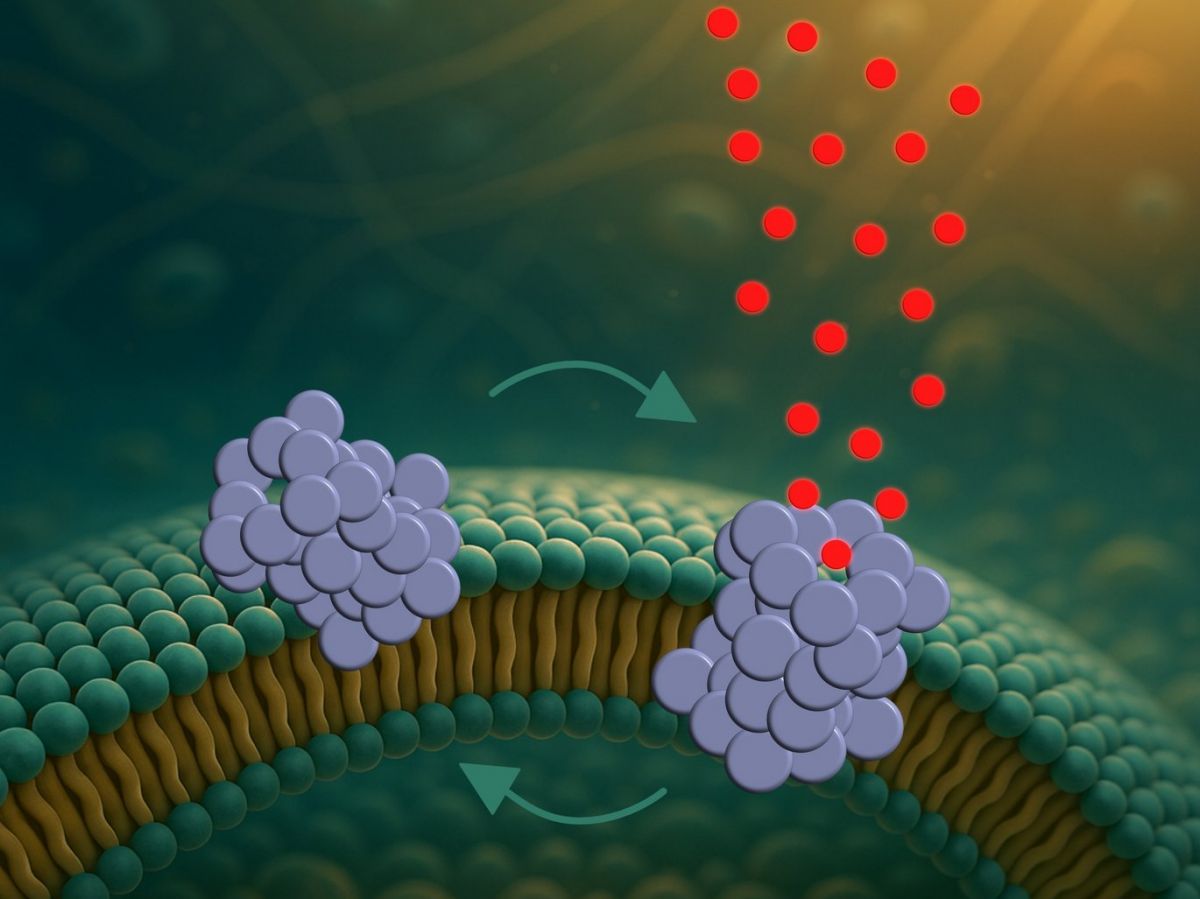
Previous studies had already shown that these oligomers ofα-synuclein are attracted to the lipids that make up cell membranes. To study this attraction and understand what the consequences might be, the Danish researchers created fake cells: vesicles formed from lipid bilayers that mimic the cell membrane. These vesicles are small artificial bubbles that mimic cell membranes and thus serve as simplified models of real cells., explains in a press release Mette Galsgaard Malle, director of the study. More than 500,000 of these liposomes were analyzed to understand how the composition of the membrane, as well as its curvature, can influence the interaction with oligomers.
These two parameters proved important for attracting α-synuclein aggregates. The more pronounced the curvature, the more these proteins adhered to the membrane lipids. But the most important thing was the lipid charge: the more negatively charged they were (which is the case for the inner walls of the cell membrane), the more they attracted the oligomers. This confirmed the attraction that pushes these aggregates toward the cell membrane.
Read alsoParkinson's disease: Researchers successfully transplant new neurons into patients
These oligomers form pores that empty the cell little by little
But what happens when these oligomers stick to the membrane? To find out, the researchers observed these interactions for five hours, taking an image every minute. The membranes, colored blue for better observation, appeared to remain stable throughout the experiment. However, some of the contents of these fake cells (colored green) escaped in fits and starts. That is, the aggregates embedded themselves in the membrane and formed pores through which the liposome contents could escape.
But these pores are not sustainable, because the leak is not continuous. So the aggregates integrate into the membrane, then come out again, and so on, gradually emptying the false cell of its contents.This dynamic could explain why in this disease cells do not die immediately, speculates Bo Volf Brøchner, author of the study. If the pores remained open, the cells would collapse very quickly. But since they open and then close, the cellular pumps could temporarily compensate for this loss.”
These aggregates ofα-synuclein would therefore weaken neurons, facilitating the emergence of the disease. However, the authors emphasize that their vesicle model is far too simple to capture the full complexity of cells, including interactions between proteins and cellular communication. Now we need to move on to the next stage and study what happens in more complex biological systems.", says Mette Galsgaard Malle. But if proven, this avenue could open up new therapeutic possibilities, attempting to prevent the formation of these pores and thus strengthen neurons, slowing the progression of the disease.