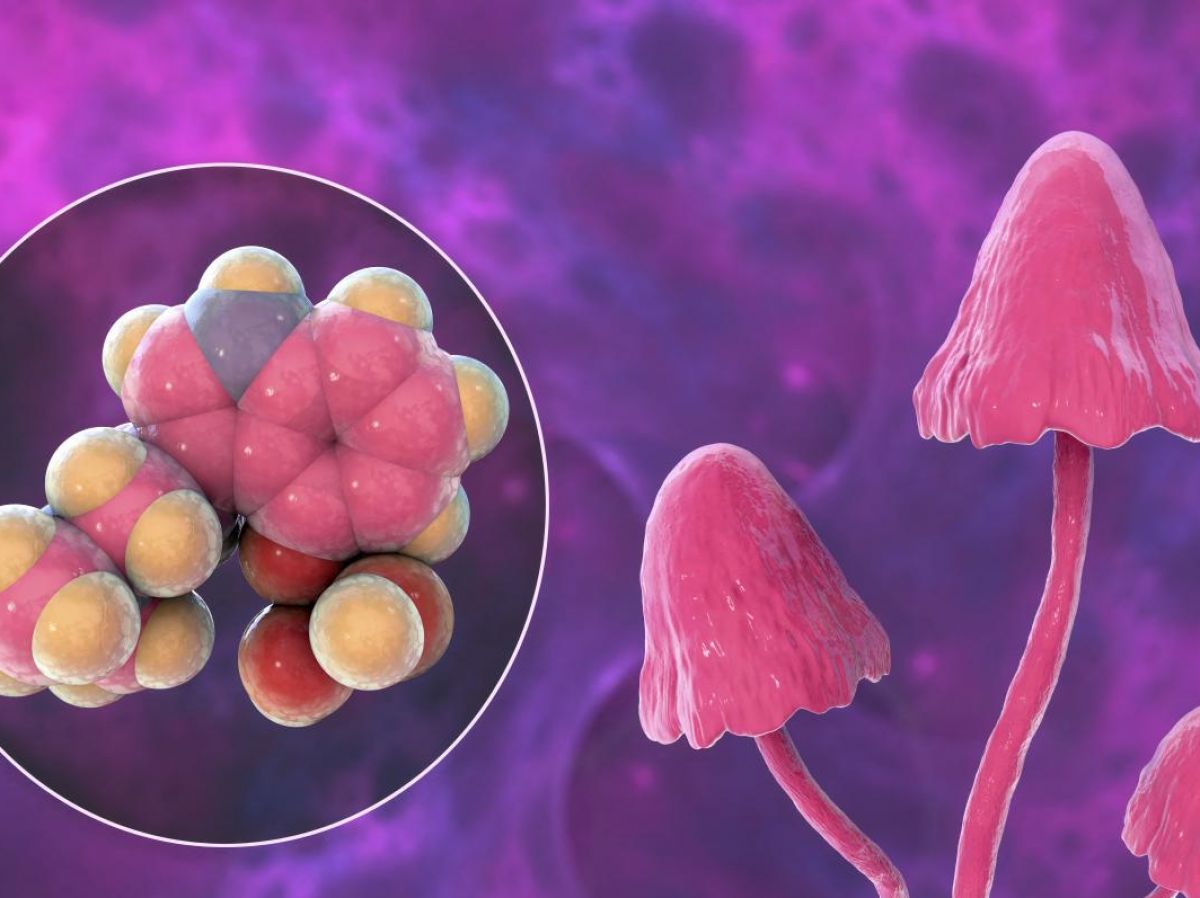
After more than 50 years of "abstinence", the year 2024 officially marks France's strong return to the race for psychedelic medicine. the pilot academic study Launched in February 2024 at the Nimes University Hospital, the clinical trial of the pharmaceutical company COMPASS Pathways began this summer at the Sainte-Anne hospital in Paris. This is the 2The eme study in France to involve the administration of psychedelic substances to humans since the 1960s.
This is an international, multi-center industrial clinical trial (taking place in several sites, including others in France which will soon follow in the footsteps of the Parisian site), in phase 3 (last phase of the drug development process before the marketing application), aimed at testing the effectiveness of the drug COMP360 against depression resistant to conventional antidepressant treatments, thanks to its active ingredient psilocybin (psychedelic substance derived from hallucinogenic mushrooms).