Press release
Thursday June 27, 2024
Monoclonal antibodies are produced from the blood of recovered patients.

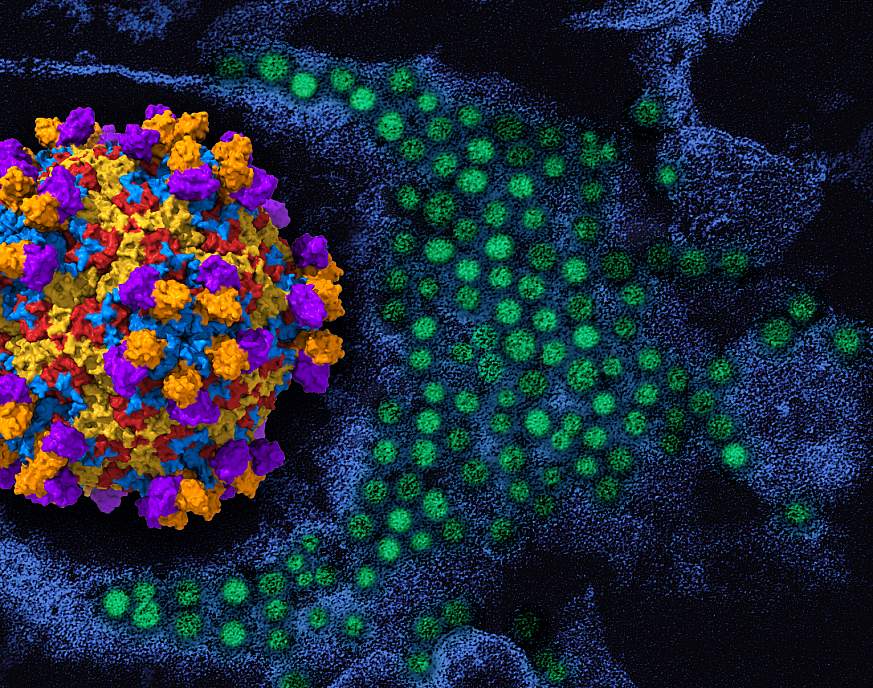
On the left, a 3D rendering of the enterovirus (red, yellow and blue viral protein), with the human monoclonal antibody EV68-228. On the left, a transmission electron microscope colorized with enterovirus D68 particles (green) is shown in the background. NIAID 3D rendering; CDC micrograph repositioned, recolored and recolored.
The National Institutes of Health is conducting a clinical study to evaluate the safety of EV-D68, which causes serious neurological and respiratory illnesses like acute flaccid myelitis (AFM), similar to polio. Scientists want to understand AFM in America, where cases have increased every two years, mostly in late summer, for the past decade. The U.S. Centers for Disease Control and Prevention (CDC) identified an increase in AFM in 2014, 2016, and 2018. EV-D68 has become a growing public health concern due to its link to intermittent outbreaks of AFM.
There are no Food and Drug Administration-approved treatments for severe EV-D68 infection or AFM. The standard of care is supportive treatments and immune disorders. These have not been evaluated in depth. EV-D68 can spread from person to person when an infected person coughs, sneezes, or touches a surface.
Scientists at Vanderbilt University Medical Center in Nashville, Tennessee, identified and isolated a neutralizing antigen, called EV68-228, from patients who recovered from EV D68 infection between 2017 and 2019. With Collaborators at Utah State University, KBio, Inc., ZabBio and ZabBio scientists then developed an experimental antigen, named EV68-228N, for testing. Monoclonal antibodies potently neutralized multiple EV-D68 clinical strains in laboratory models over multiple epidemic years. Kbio, Inc. uses its plant-based protein development platform to manufacture EV68-228N.
The Phase I study, sponsored by the NIH National Institute of Allergy and Infectious Diseases, will be led by C. Buddy Creech MDMPH of Vanderbilt University Medical Center. It will evaluate the safety of EV68-228N, how long it lasts in the body and the most effective dosage. E. Adrianne Hammershaimb will lead the trial at the University of Maryland in Baltimore. This study will be conducted by academic medical centers across the United States in partnership with the Infectious Disease Research Consortium funded by NIAID. NIAID funds vaccine and treatment evaluation units.
Clinical test NCT06444048 The study will involve 36 healthy people aged 18 to 49. Six volunteers will receive a placebo as a control group, and 30 others, divided into groups of 10, will each receive a dose of 3, 10 or 30 mg/kg of EV68-228 N intravenously. Scientists will assess the safety of the experimental treatments by monitoring the first two study participants in each group for 72 hours before further infusions. Researchers will monitor and evaluate study participants during nine in-person exams over the next 120 days.
EV-D68, according to the CDC, was first identified in California in 1962. It is one of more than 100 enteroviruses that do not cause polio. EV-D68 is generally mild and causes respiratory illness. Non-polio enteroviruses can be very common. The majority of infections cause mild or no symptoms. These include a runny and itchy nose, sneezing and coughing, as well as rashes, mouth sores, muscle pain and aches. Wheezing or difficulty breathing are serious symptoms.
In 1987, doctors and health officials began reporting sporadic cases of EV D68 to the CDC. Between August and December 2014, EV D68 was responsible for an outbreak of respiratory illness in the United States and 120 cases of AFM in 34 states. This has increased awareness of EVD68 and its associated disease. In 2014, CDC surveillance of the EVD68 virus was expanded. Cases of EV-D68 and AFM have been detected annually in the United States, primarily in late summer or early fall, but with pronounced increases in 2016 and 2018
NIAID supports and conducts research at NIH, the United States, and internationally to study and develop new methods to diagnose, prevent, and treat infectious and immune-mediated diseases. On the NIAID website, you can find press releases, fact sheets, and other NIAID materials. NIAID website.
The National Institutes of Health: NIH is the medical research agency of the United States Department of Health and Human Services. It includes 27 institutes and centers. The NIH, the nation's medical research agency, is comprised of 27 institutes and centers and is part of the U.S. Department of Health and Human Services. Visit NIH for more information about its programs. www.nih.gov.
NIH…Transforming Discovery into Healthcare(r)
###
