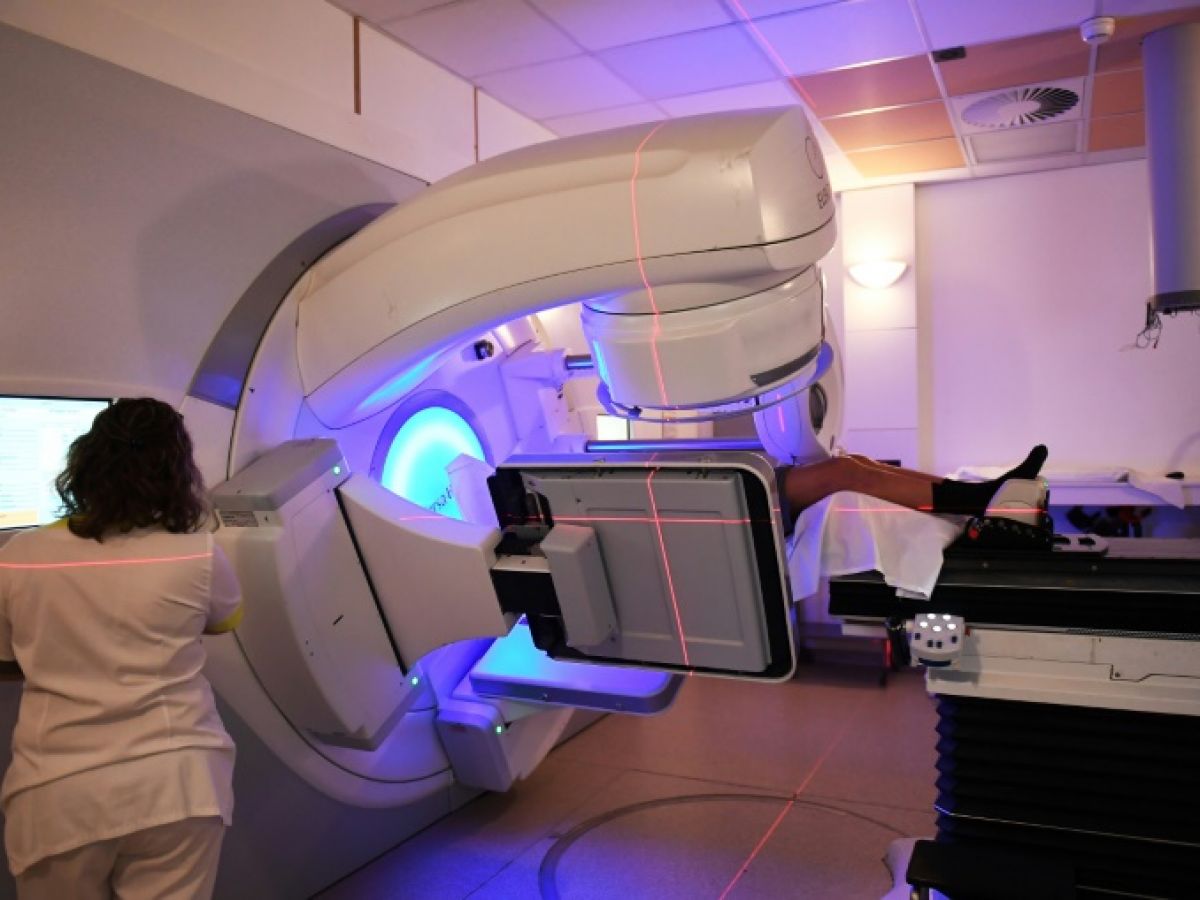
Injecting radioactive components into the body that will directly destroy cancer cells. This approach still has only a small place in the arsenal of cancerologists, but it appears increasingly promising and the pharmaceutical industry is now investing billions of euros in it.
These radioactive drugs are " in the air of time", summarizes to AFP the financial analyst Jamila El Bougrini, specialist in the pharmaceutical sector. A recent operation between two French giants testifies to this. Announced in mid-October, a partnership will bring together the pharmaceutical group Sanofi and the nuclear specialist Orano, the former Areva.
Passed largely unnoticed, in a context where Sanofi is at the heart of a controversy surrounding the sale of its subsidiary manufacturing Doliprane, the announcement is somewhat surprising. What do boxes of medicines and Orano's nuclear power plants have in common? The answer lies in the very concept of nuclear medicine and, more specifically, of unique medicines. Orano is developing one of them through a subsidiary in which Sanofi will take a small stake for 300 million euros.
Hitting cancer cells directly
These drugs use radioactive components to destroy tumors. To do this, they are combined with a molecule capable of identifying typical markers of a cancer cell and, therefore, serving as a "vector" to carry the radioactive element straight to it.
The general principle is well known since it is that of the Radiotherapy, which already treats the majority of cancer patients. But instead of emitting rays onto the person, the aim here is to directly hit the cancer cells, with the promise of very high precision. We are therefore talking about radiotherapy " Targeted“.
In a broad sense, the concept has already been used for decades in cancerology since radioactive iodine is regularly used to treat certain thyroid cancers. But the case of iodine is special, because it is naturally attracted to the thyroid and therefore does not require an associated "vector". On the other hand, for several years, therapies have produced results by successfully combining a radioactive component and a biological vector.
And the pharmaceutical industry follows. The big turning point was the acquisition in 2018 by the Swiss Novartis, for almost four billion dollars, of a radioactive treatment, Lutathera. Before, no one was interested in our discipline", recognizes to the AFP Francoise Kraeber-Bodere, specialist in nuclear medicine at the University Hospital of Nantes.
Industry rushes
However, Lutathera is limited to rare cancers of the digestive system. It was shortly after that Novartis opened a " mass march", in the words of Ms. Kraeber-Bodere, with the purchase for two billion dollars of another radiotherapy, Pluvicto, against certain prostate cancers, and the publication of positive data in the early 2020s.
Since then, the pharmaceutical giants have been rushing in. In recent months, AstraZeneca, Bristol-Myers Squib (BMS), Eli Lilly and, once again, Novartis have all paid billions of dollars to buy biotechs specializing in targeted radiotherapy. The merger between Sanofi and Orano is the latest example of this excitement. Has the French giant, which has also been criticized for its ability to innovate since its slowness in developing an anti-Covid vaccine, simply followed the movement?
“ Sanofi's operation is not pioneering, but it places the group among the most advanced players in radiotherapy.", qualifies Mrs. El Bougrini. Indeed, the drug developed by Orano, based on lead 212, belongs to a new generation of radiotherapies, based on so-called alpha particles. These release more radioactive energy than beta particles, the basis of most existing therapies, but over a shorter distance. This therefore gives hope for an even more selective mode of action.
It remains to be seen whether this promise will actually translate into convincing clinical results. Most alpha therapies have not yet given rise to large-scale trials, a step that Orano is preparing to take. While remaining cautious, specialists are optimistic: " The first clinical results tell me that it will be effective.Mrs. Kraeber – Bodere: “ If I had been asked five years ago, I would have said that it was still a bit risky. Now I think we have to go for it.“