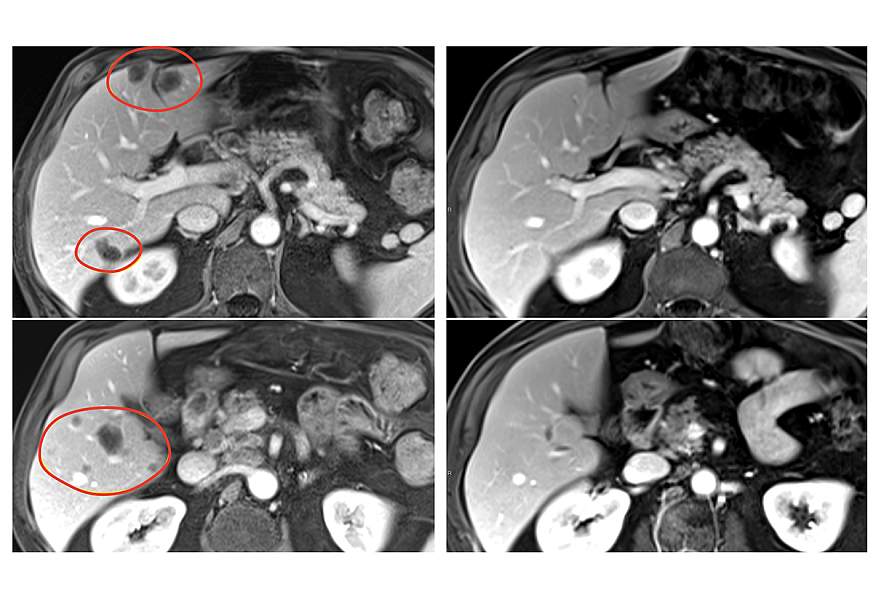
Combination immunotherapy reduced a variety of metastatic gastrointestinal cancers
April 1, 2025
Press release
Tuesday, April 1, 2025
An NIH trial shows that a new form of TIL therapy is effective against tumors of the colon, rectum, pancreas, and bile ducts.

A new form of tumor-infiltrating lymphocyte (TIL) therapy, a form of personalized cancer immunotherapy, has significantly improved treatment effectiveness in patients with metastatic gastrointestinal cancers, according to results of a clinical trial led by researchers at the National Institutes of Health (NIH). The findings, published April 1, 2025, in Nature Medicine, give hope that this therapy could be used to treat a variety of solid tumors, something that has so far eluded researchers developing cell therapies.
This form of therapy involves identifying and selecting immune cells (TILs) within the tumor that specifically recognize and attack the patient's tumor cells. Scientists then grow these TILs in large quantities in the laboratory before administering them to the patient.
Patients in the clinical trial, with various gastrointestinal tumors, also received the immune checkpoint inhibitor pembrolizumab (Keytruda) to further boost their immune response. The result was that nearly 24% of patients treated with selected TILs plus pembrolizumab experienced substantial tumor reduction, compared with 7.7% of patients receiving selected TILs without pembrolizumab. Patients treated with TILs not selected for antitumor activity experienced no tumor reduction.
“We are seeing the first expansion of TIL cell therapy to common solid cancers,” said Steven A. Rosenberg, MD, PhD, principal investigator of the study at the National Cancer Institute of the NIH. “We are seeing a small crack in the solid cancer wall using cellular immunotherapy for common solid cancers, and we believe we have ways to open it even further.”
The clinical trial included 91 patients with metastatic gastrointestinal cancers—including esophageal, gastric, pancreatic, colon, and rectal cancers—who had worsened despite a median of four prior treatment regimens. In the pilot phase of the trial, 18 patients were treated with TILs that had not been selected for their antitumor activity, and there were no objective responses (tumor shrinkage of at least 30 % is considered an objective response). In the second phase, 39 patients were treated with selected TIL therapy, and three (7.7 %) had objective responses.
In the third phase, 34 patients received pembrolizumab immediately before the selected TIL therapy to prevent the newly introduced immune cells from being inactivated by their own immune system. This group had the best response, with 8 of 34 patients (23.5 %) experiencing an objective response. All 91 patients had also received standard chemotherapy and high-dose interleukin-2 before TIL treatment.
In the second and third phases of the trial, objective responses were observed in several types of gastrointestinal cancers, including colon, rectal, pancreatic, and biliary tract cancers. Responses lasted between 8 months and more than 5.8 years in the group receiving selected TIL therapy alone, and between 4 months and 3.5 years in the group receiving selected TIL therapy and pembrolizumab. Serious side effects occurred in 30 % patients treated with selected TILs.
Researchers are currently developing methods to identify TILs that recognize multiple specific proteins within a tumor, called neoantigens, to help increase the number of patients who respond to selected TIL treatment with pembrolizumab.
TIL therapy, developed in the late 1980s by Dr. Rosenberg and his colleagues at the NIH, uses an individual's own TILs to fight their tumor cells. Last year, the Food and Drug Administration approved the first TIL therapy for a solid cancer, lifileucel (Amtagvi), for the treatment of advanced melanoma.
The new study was co-led by Dr. Rosenberg and NCI researchers Frank J. Lowery, Ph.D., and Stephanie L. Goff, MD
About the National Cancer Institute (NCI): The NCI leads the National Cancer Program and the NIH's efforts to significantly reduce cancer prevalence and improve the lives of people with cancer. The NCI supports a wide range of cancer research and training outside the NCI's walls through grants and contracts. The NCI's intramural research program conducts innovative, transdisciplinary basic, translational, clinical, and epidemiological research on cancer causes, prevention, risk prediction, early detection, and treatment, including research at the NIH Clinical Center, the world's largest research hospital. Learn more about intramural research conducted at the NCI Center for Cancer Research. For more information about cancer, please visit the NCI website at cancer.gov or call the NCI Contact Center at 1-800-4-CANCER (1-800-422-6237).
About the National Institutes of Health (NIH): The NIH, the nation's medical research agency, comprises 27 institutes and centers and is part of the U.S. Department of Health and Human Services. The NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and investigating the causes, treatments, and cures for common and rare diseases. For more information about the NIH and its programs, visit www.nih.gov.
NIH…Transforming Discovery into Health®
###

